Understanding the IB Chemistry Data Booklet
If you’re aiming for a Level 6 or 7 in HL Chemistry, you need to treat the Data Booklet like an extension of your notes.
The IB Chemistry Data Booklet is an essential tool for both Standard Level (SL) and Higher Level (HL) students—especially in Paper 2 and Paper 3. But despite being allowed in the exam room, many students only glance at it during their revision, missing out on its full value.
This guide explains how the Data Booklet works, what’s inside the HL version, how to use it effectively in your IB Chemistry exams, and what examiners expect you to do with it.
What Is the IB Chemistry Data Booklet?
The IB Chemistry Data Booklet is a reference document provided to students during their final exams. It includes:
Constants and formulas
Periodic table
Electrochemical series
Spectral data
Standard enthalpies and bond energies
Tables and diagrams essential for calculations and analysis
The booklet is the same for SL and HL students, but HL candidates are often expected to make more sophisticated use of it—particularly in Paper 3, which involves experimental and data-based problem solving.
When Can You Use the Data Booklet?
You can use the booklet in:
Paper 2 (structured questions)
Paper 3 (data-based and experimental questions)
You cannot use it in:
Paper 1 (multiple choice), which is done without any reference materials
The booklet is available online through the IB’s official website, and your teacher will likely provide it throughout the course. If you're studying independently, download the most recent version and print a hard copy to practise with during revision.
Why HL Students Must Understand the Booklet Deeply
At HL, you’ll encounter more complex:
Calculations
Equilibrium and kinetics problems
Spectroscopy and organic chemistry questions
Multi-step data analysis in Paper 3
You’ll be expected not only to locate the right values, but also to know when and how to apply them with precision.
In other words, the Data Booklet isn't just a safety net—it's a toolkit. And using it fluently can save you time and earn you marks.
What’s Inside the IB Chemistry Data Booklet?
Let’s walk through the main sections and highlight what you should know as an HL student:
1. Useful Constants and Units
This includes:
Avogadro’s constant (L)
Gas constant (R)
Specific heat capacity of water
Speed of light
Planck’s constant
Use it for:
Molar calculations
Energetics and enthalpy changes
Spectroscopy (e.g. E = hν)
💡 HL Tip: Learn how these constants relate to equations in energetics and quantum theory. They’re not just for plugging into formulas—they’re part of your conceptual toolkit.
2. Formulae and Equations
Covers:
Ideal gas law
Gibbs free energy (ΔG = ΔH – TΔS)
Equilibrium constants
Beer–Lambert law
Rate equations
Use it for:
All multi-step calculation questions
Thermodynamics, kinetics, and equilibrium challenges
💡 HL Tip: In Paper 2, HL questions often combine these formulas (e.g. calculate K from ΔG). Be ready to link sections.
3. The Periodic Table
A classic—but often underused. Includes:
Element symbols, atomic numbers
Relative atomic masses
Periods and group layout
Use it for:
Calculating molar masses
Predicting chemical trends (atomic radius, electronegativity, etc.)
Identifying block/period/group for bonding questions
💡 HL Tip: Use the periodic table to analyse ionisation trends and electron configurations. You may need this to explain anomalies or predict reactivity.
4. Electrochemical Series
Lists:
Standard electrode potentials (E⁰)
Redox couples (e.g. Zn²⁺/Zn)
Use it for:
Calculating cell potentials (Ecell = Ereduction – Eoxidation)
Identifying oxidising and reducing agents
Predicting whether redox reactions will occur spontaneously
💡 HL Tip: Master how to combine standard potentials with ΔG using ΔG = –nFE. It’s a common HL Paper 2 or 3 question.
5. Bond Enthalpies and Lattice Enthalpies
Includes:
Average bond enthalpies (in kJ mol⁻¹)
Data tables for bond types (e.g. C–C, O–H, N≡N)
Use it for:
Calculating approximate ΔH of reactions using bond enthalpies
Evaluating exothermic/endothermic reactions
💡 HL Tip: Be able to compare bond enthalpy methods vs. Hess’s Law. This often appears in Paper 2 HL questions asking for evaluation of methods.
6. Enthalpies of Combustion and Formation
Tables showing standard enthalpies (ΔHc°, ΔHf°) for many substances.
Use it for:
Constructing Hess cycles
Calculating enthalpy changes of reactions
Evaluating enthalpy changes in combustion reactions
💡 HL Tip: You may be asked to comment on experimental vs. theoretical values—this is often linked to internal assessment and practical design too.
7. IR Spectral Data
Table showing functional group absorptions (in cm⁻¹):
O–H stretch
C=O stretch
N–H bend, etc.
Use it for:
Interpreting IR spectra in organic chemistry
Identifying functional groups in unknown compounds
💡 HL Tip: Pair IR data with 1H NMR data to build full structural assignments. This integrated approach is expected in HL Paper 3.
8. 1H NMR Chemical Shift Values
Includes:
Chemical shift ranges (δ) for various hydrogen environments
Splitting patterns and relative intensity guidance
Use it for:
Determining molecular structure
Explaining NMR spectra in synthesis or identification questions
💡 HL Tip: This is a core HL organic skill. You’ll often be asked to assign structure based on IR + NMR + molecular formula. Be confident navigating all three.
9. Colour of Complex Ions
Includes colours of transition metal complexes:
[Cu(H₂O)₆]²⁺ – blue
[Fe(H₂O)₆]³⁺ – yellow/brown
[Ni(H₂O)₆]²⁺ – green
Use it for:
Explaining colour changes in ligand substitution
Describing d-orbital splitting and transition metal properties
💡 HL Tip: Understanding crystal field theory is a key HL-only concept. This section supports Paper 2 and Paper 3 questions on d-block chemistry.
10. Ka and pKa Values
Includes dissociation constants for weak acids.
Use it for:
Calculating pH of weak acids
Buffer calculations
Comparing acid strength
💡 HL Tip: HL students should be fluent with Ka, Kb, and buffer systems. Many HL questions ask you to explain buffer action using both pH and equilibrium logic.
How to Practise Using the Data Booklet
It’s not enough to simply have the booklet—it must become second nature.
Here’s how to practise:
Use it during every past paper you attempt
Highlight sections you use most often (in your printed copy)
Create practice questions that require using a specific table (e.g. “Calculate Ecell using the electrochemical series”)
Time yourself to speed up data location
Use tabs or colour-coding (if your teacher allows this for practice—note: not allowed in the real exam)
Common Mistakes Students Make with the Data Booklet
❌ Not using the Data Booklet in Paper 2 practice
❌ Forgetting to cross-reference values like bond enthalpies
❌ Misreading units or interpreting molar values incorrectly
❌ Using the wrong data table for a calculation
❌ Not checking whether values are standard state or average
Avoid these by building familiarity now, not in the last week before the exam.
Using the Data Booklet in Internal Assessment (IA)
The Data Booklet can also be helpful during your IA planning and evaluation stages:
Refer to standard enthalpy or Ka values in your discussion
Use constants and units for better calculation accuracy
Support analysis with theoretical expectations
Quoting relevant values from the booklet shows examiner-level thinking and strengthens your analysis section.
Final Thoughts: The Data Booklet Is a Tool, Not a Crutch
If you’re aiming for a Level 6 or 7 in HL Chemistry, you need to treat the Data Booklet like an extension of your notes. It’s there to support your answers—but it won’t do the thinking for you.
Mastering how to use it:
Saves time in the exam
Helps avoid silly calculation errors
Allows you to focus on logic and structure
Impresses examiners with precision and independence
Need Help Using the Data Booklet Strategically?
Book a 15 mins consultation with Dr Marguerite Quinn, an experienced IB Chemistry tutor who teaches students how to use the Data Booklet effectively—during revision, in the IA, and in exam conditions. One small change in your exam technique can lead to big improvements in your final grade.


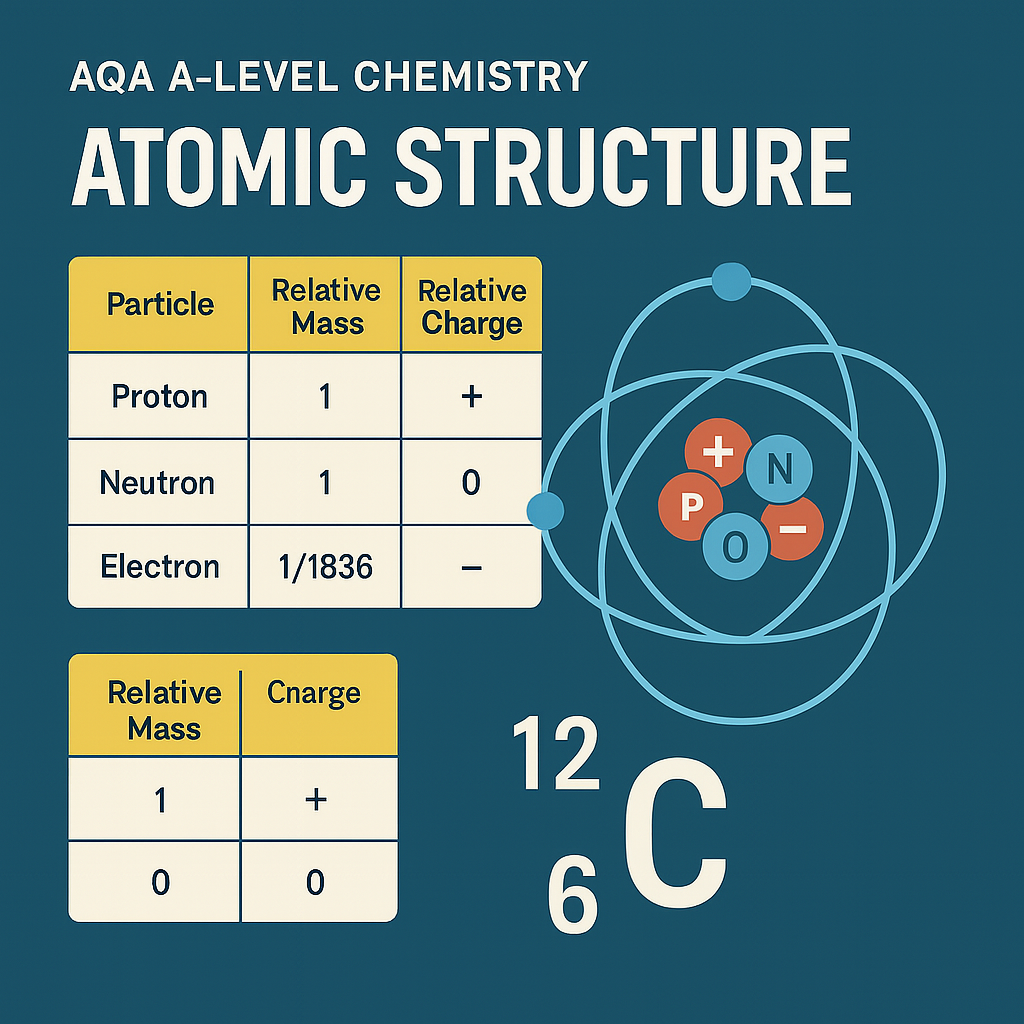


Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.