Top 10 Past Paper Questions Students Get Wrong in Chemistry
If you’re making these mistakes — you’re not alone. These questions trip up thousands of students each year.
You’ve revised, made flashcards, and gone over the specification. But when it comes to past paper practice, certain Chemistry questions trip up even the best students.
Whether you’re studying for GCSE or A-Level, these common question types appear year after year — and students frequently lose marks not because they don’t know the topic, but because they misread, misinterpret, or miss the mark scheme’s expectations.
Here are the top 10 past paper questions students regularly get wrong — and how to get them right.
1. Bond Angles and Molecular Shapes
The mistake: Naming the wrong shape or giving incorrect angles for molecules like NH₃, CH₄, or H₂O.
Why it happens: Students forget the impact of lone pairs on bond angles or mix up names like "trigonal planar" and "trigonal pyramidal."
Fix it:
Use a table to memorise common shapes and angles.
Always state both the shape and reason (e.g. “4 bonding pairs, no lone pairs → tetrahedral, 109.5°”).
2. Ionic vs Covalent Bonding Descriptions
The mistake: Mixing up bonding types or giving vague answers like “they share electrons.”
Why it happens: Lack of precision in definitions or confusion between particle types.
Fix it: Learn the exact language:
Ionic = “Electrostatic attraction between oppositely charged ions.”
Covalent = “Shared pair of electrons between non-metal atoms.”
3. Enthalpy Change Calculations (ΔH)
The mistake: Getting the wrong sign (+/-), incorrect units, or forgetting to divide by moles.
Why it happens: Students skip steps or don’t apply ΔH = q ÷ n correctly.
Fix it:
Write the formula every time.
Use correct units (kJ mol⁻¹), and check whether it’s exothermic or endothermic.
4. Equilibrium Shifts (Le Chatelier’s Principle)
The mistake: Giving vague answers like “it moves to the right” without stating why.
Why it happens: Students fail to link changes to the system (e.g. pressure, temperature) to the position of equilibrium using clear reasoning.
Fix it: Always mention:
The change (e.g. increased temperature)
The direction of shift
The reason (e.g. “forward reaction is endothermic”)
The effect on yield
5. Writing Full and Ionic Equations
The mistake: Including spectator ions or unbalanced equations.
Why it happens: Rushing or misunderstanding what ionic equations are asking for.
Fix it:
Always balance both mass and charge.
Cancel out spectator ions.
Practice common reactions (precipitation, displacement, acid-base).
6. Percentage Yield & Atom Economy
The mistake: Using the wrong formula or confusing the two concepts.
Why it happens: Students memorise steps but don’t fully understand what each term means.
Fix it:
% yield = (actual ÷ theoretical) × 100
Atom economy = (Mr of desired ÷ total Mr of products) × 100
Label your numbers clearly to avoid flipping them.
7. Redox Reactions and Oxidation Numbers
The mistake: Assigning incorrect oxidation states or not explaining redox changes fully.
Why it happens: Skipping steps or forgetting the rules for assigning oxidation numbers.
Fix it:
Practise rules for O, H, group numbers
Identify what's oxidised and reduced
Use correct terms: oxidation = loss of electrons / increase in oxidation number
8. Required Practical Questions
The mistake: Giving method steps out of order or missing key terms like “control variables.”
Why it happens: Not reviewing the specific practicals ahead of time.
Fix it:
Go through all required practicals for your board
Learn the purpose, method, expected results, and safety considerations
Use past paper wording to practise
9. Nomenclature and Functional Groups (Organic Chemistry)
The mistake: Misnaming compounds, incorrect numbers or missing the functional group altogether.
Why it happens: Lack of practice or misunderstanding IUPAC rules.
Fix it:
Name longest chain first
Identify and number the functional group
Practise with structural and skeletal formulae
10. Energy Level Diagrams and Activation Energy
The mistake: Drawing exothermic/endothermic curves incorrectly, or forgetting to label Ea (activation energy).
Why it happens: Confusion over diagrams or rushing the drawing.
Fix it:
Show reactants and products with energy levels clearly
Label activation energy and enthalpy change (ΔH)
Use arrows and dotted lines to annotate
Final Thought
If you’re making these mistakes — you’re not alone. These questions trip up thousands of students each year, but once you understand why, they become easier to fix.
The key is exam technique: knowing what the mark scheme wants, avoiding vague language, and practising under timed conditions.
Want help improving your past paper performance? Book a consultation and let’s create a plan towards top marks.


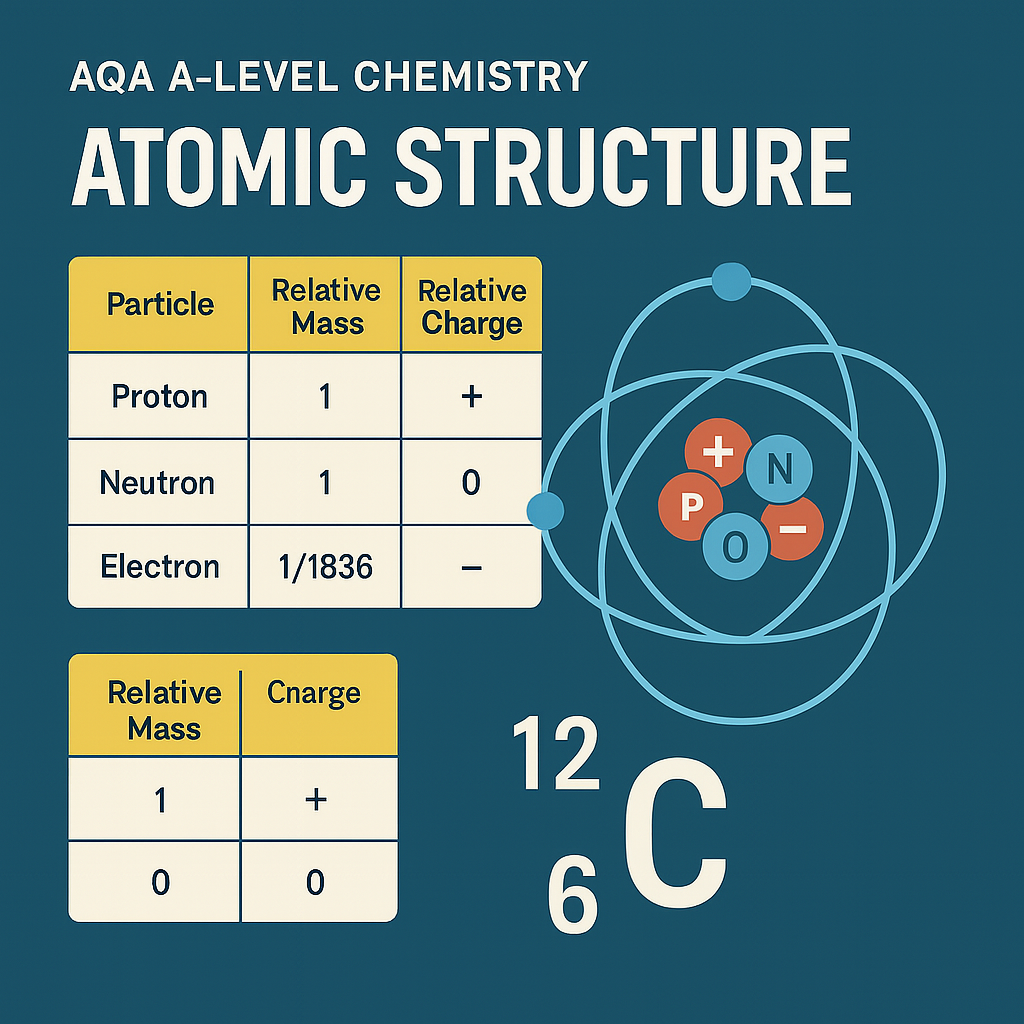


Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.