Essential Maths Skills for GCSE Chemistry: A Complete Student Guide
Chemistry is a quantitative science. It’s not enough to say that something reacts—you often need to show how much, how fast, or what proportion.
GCSE Chemistry is about more than just reactions, equations, and the periodic table—it’s also about numbers. In fact, around 20% of the marks in your Chemistry exam come from mathematical skills. That’s why getting to grips with the maths side of Chemistry is essential if you’re aiming for a strong grade—especially a 7, 8 or 9.
Whether you're a natural with numbers or find maths more of a challenge, this guide will walk you through the essential skills you need, how they appear in exam questions, and how to practise them effectively.
Why Maths Skills Matter in Chemistry
Chemistry is a quantitative science. It’s not enough to say that something reacts—you often need to show how much, how fast, or what proportion.
GCSE Chemistry questions regularly ask you to:
Calculate moles, masses, or concentrations
Work with ratios and formulae
Analyse graphs or data tables
Convert units (e.g. cm³ to dm³)
Apply formulae like pH = –log[H⁺] or Q = mcΔT
You don’t need to be a maths genius. But you do need to:
Understand what the question is asking
Pick the correct method
Lay out your working clearly
Use the right number of significant figures or decimal places
Let’s break down the skills.
1. 🧪 Using Formulae Correctly
This underpins almost every Chemistry calculation.
Example:
Formula: moles = mass ÷ Mr
You need to:
Rearrange if the question gives you different values
Use the correct relative atomic mass (Ar) or Mr (from the periodic table)
Include correct units (e.g. g/mol, mol)
Top Tips:
Memorise core equations (they’re not all on the formula sheet)
Practise rearranging simple equations with confidence
Always check units before plugging in numbers
2. ⚖️ Moles, Mass, and Mr
This is one of the most common calculation areas at GCSE.
What you’ll need:
Use the triangle: mass = moles × Mr
Understand how to find Mr from a chemical formula (e.g. H₂O = 2×1 + 16 = 18)
Example Question:
What is the mass of 0.5 mol of water?
Step-by-step:
Mr of H₂O = 18
mass = 0.5 × 18 = 9g
💡 Always show your working—it can earn method marks even if your final answer is wrong.
3. 🧮 Percentage Composition and Empirical Formula
Key Skills:
Calculate percentage by mass:
% = (Ar of element ÷ Mr of compound) × 100
Find empirical formula:
Convert % to grams
Divide by Ar
Divide by smallest value
Write formula in simplest ratio
Example:
A compound contains 12g of carbon and 32g of oxygen. What is the empirical formula?
C: 12 ÷ 12 = 1
O: 32 ÷ 16 = 2
Empirical formula = CO₂
4. 📈 Interpreting Graphs and Tables
You’ll often be given a graph showing:
Rates of reaction
Temperature changes
Gas volume over time
Or a table with:
pH values
Masses before/after reaction
Concentration values
What you need to do:
Extract values accurately
Estimate from a curve if needed
Calculate gradients (for rate of reaction)
Describe trends or make comparisons
Example:
Describe how the rate of reaction changes over time.
Answer: “The rate is fastest at the start (steepest gradient) and decreases over time as the reactants are used up.”
📉 Be prepared to describe, compare and explain—this brings together maths and science.
5. 📏 Units and Conversions
GCSE Chemistry questions love to test whether you:
Convert cm³ to dm³ (1000 cm³ = 1 dm³)
Convert between g, kg, mg
Use mol/dm³ for concentration
Know that 1 mol of gas = 24 dm³ (at room temp)
Top Tip:
Always write units in your working. If you get the wrong unit, you could lose marks even if the number is right.
6. 💧 Concentration Calculations
One of the most frequently tested skills.
Formula:
concentration = moles ÷ volume (dm³)
Example:
You dissolve 0.2 mol of NaCl in 0.5 dm³ of water. What is the concentration?
concentration = 0.2 ÷ 0.5 = 0.4 mol/dm³
You might also be given:
Mass and asked to find moles
Volume in cm³ (convert to dm³)
The number of moles and asked to calculate volume
7. Reacting Masses and Limiting Reactants
You’ll often be asked to:
Predict how much product will be formed
Work out how much of a reactant is needed
Identify the limiting reactant
Steps:
Write balanced equation
Calculate moles of given substance
Use ratio from equation
Calculate mass or volume of unknown
Example:
2H₂ + O₂ → 2H₂O
How much water is made from 4g of hydrogen?
Moles of H₂ = 4 ÷ 2 = 2 mol
Ratio: 2 mol H₂ → 2 mol H₂O
Moles of H₂O = 2
Mr of H₂O = 18 → mass = 2 × 18 = 36g
8. Energy Changes (Q = mcΔT)
This formula crops up in required practicals and Paper 2:
Q = mcΔT
Where:
Q = heat energy (Joules)
m = mass of water (g)
c = specific heat capacity (usually 4.18 J/g°C)
ΔT = change in temperature
Example:
100g of water is heated, and its temperature increases by 20°C. What is the energy transferred?
Q = 100 × 4.18 × 20 = 8360 J
9. Percentage Yield and Atom Economy
This is all about efficiency and sustainability.
Formulae:
Percentage yield = (actual ÷ theoretical) × 100
Atom economy = (Mr of desired ÷ total Mr of all products) × 100
Example:
In a reaction, 25g of product was made. The theoretical yield was 30g.
% yield = (25 ÷ 30) × 100 = 83.3%
♻️ These calculations are often tied to 6-mark questions asking you to explain why a process is efficient or not.
10. pH and Logarithms (Higher Tier)
For higher tier students, you may need to use:
pH = –log[H⁺]
This will usually be calculator-based.
Example:
The H⁺ concentration is 1 × 10⁻³ mol/dm³. What is the pH?
pH = –log(1 × 10⁻³) = 3
You might also be asked to:
Compare two pH values
Estimate how much more acidic one solution is than another
📝 Exam Technique Tips for Chemistry Maths Questions
Always write the formula first—you may get a mark just for that
Show every step of your working clearly
Underline units in the question and check them in your answer
Use a calculator where appropriate—avoid mental maths
Round carefully—2 or 3 significant figures is often expected
Label your answer with the correct unit (g, mol, dm³, etc.)
🧠 How to Practise Chemistry Maths Skills Effectively
Use exam-board specific questions (AQA, OCR, Edexcel)
Create a maths formula sheet to revise from regularly
Practise under timed conditions to improve speed
Mark your answers using the official mark scheme
Keep a “mistake log”—note down where you went wrong and how to fix it
Use visual tools like triangle formulas, flow charts, and worked examples
🔁 Core GCSE Chemistry Formula List to Memorise
Core GCSE Chemistry Formula List to Memorise
🎓 Final Thoughts: Master the Numbers, Master the Grade
You don’t need to love maths to succeed in Chemistry. But by learning the core skills, practising consistently, and building your confidence step by step, you’ll be able to tackle any calculation question that comes your way.
And remember: every calculation mark you get moves you closer to that grade 7, 8, or 9.
Need Support With Chemistry Calculations?
Dr. Marguerite Quinn is a PhD-qualified Chemistry tutor who specialises in helping students develop maths confidence and exam-ready calculation skills—whether you’re aiming to pass or push for the top grades.
👉 Book a 15 mins consultation today to find out how 1:1 online Chemistry tutoring can help you turn number worries into exam wins.dent’s pace, preferences, and learning profile. Her calm, visual teaching style has helped students with ADHD, autism, dyslexia, and anxiety find success and confidence in Chemistry.



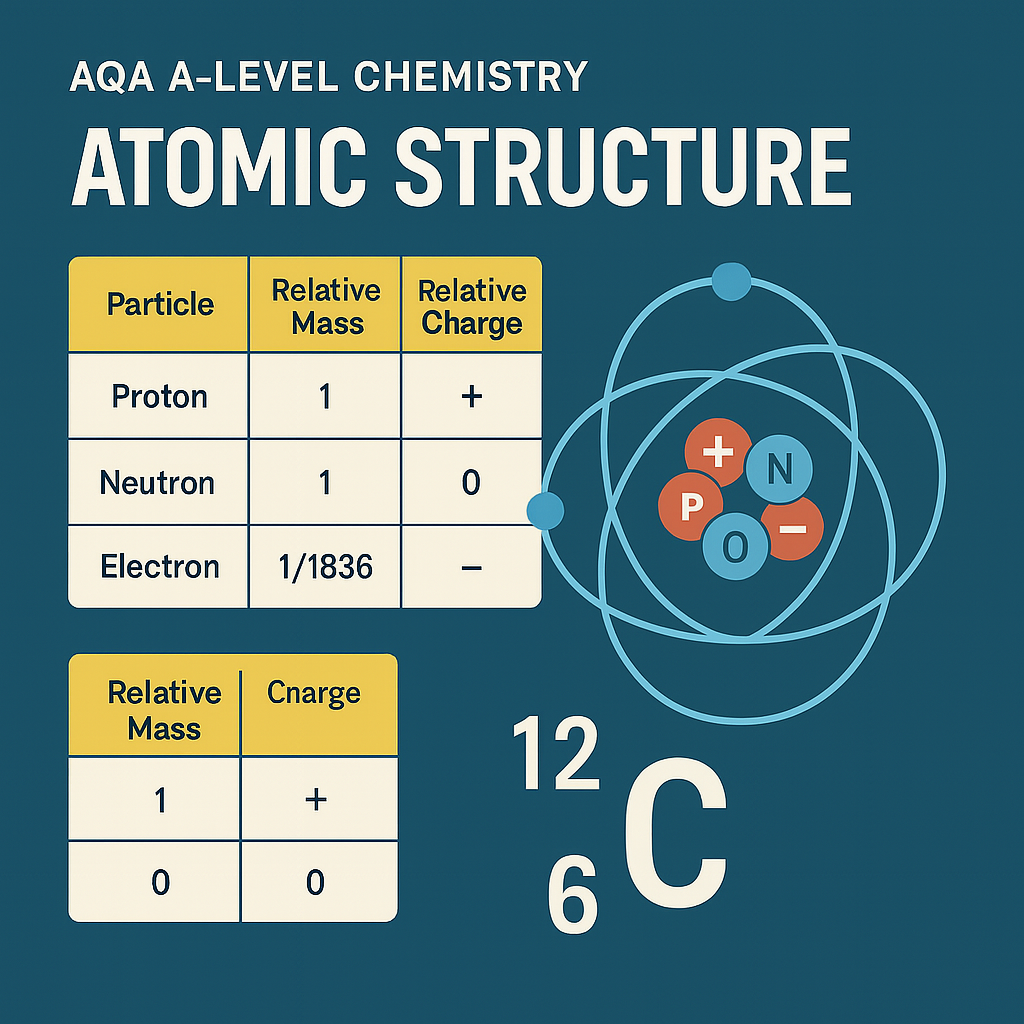


Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.