Essential Maths Skills for IB Chemistry: A Student Guide for SL and HL
Since so many marks depend on both doing the maths and explaining it clearly, mastering these skills gives you a major advantage.
Essential Maths Skills for IB Chemistry: A Student Guide for SL and HL
The International Baccalaureate (IB) Chemistry course is rigorous and rewarding—but it comes with a serious side of numbers. Whether you're studying Standard Level (SL) or Higher Level (HL), mathematical skills play a critical role in your ability to succeed in assessments, especially Paper 2 and the Internal Assessment (IA).
This guide breaks down the key maths skills you need for IB Chemistry, how they show up in exams, and how to build your confidence to handle them effectively—whether you're aiming for a solid pass or pushing for a level 7.
Why Maths Matters in IB Chemistry
Mathematical understanding is explicitly listed as one of the Assessment Objectives in the IB Chemistry syllabus. While IB Chemistry is not a mathematics course, your ability to apply maths within a chemistry context is assessed throughout.
Maths is used to:
Perform chemical calculations
Interpret and analyse data from experiments
Manipulate units, significant figures, and orders of magnitude
Represent chemical relationships graphically
Evaluate uncertainties and errors in practical work
For HL students, the mathematical demand is higher, with more frequent and complex calculations.
10 Essential Maths Skills for IB Chemistry (SL & HL)
Let’s look at the most important maths topics you need to master.
1. Moles, Mass, and Mr
Formula: mol = mass ÷ Mr
You must be able to:
Calculate moles from mass and molar mass
Use molar mass units (g/mol)
Rearrange formulae when needed
This appears early in the syllabus (Stoichiometry) and underpins many later topics like titrations and gas laws.
2. Solutions and Concentrations
Formula: concentration = mol ÷ volume (in dm³)
You’ll often be given volumes in cm³, so remember to convert to dm³ by dividing by 1000.
Titration questions commonly require you to:
Calculate moles from one solution
Use mole ratios to calculate concentration or volume of the other
Show clear, logical working
Tip: Always label your units and round your final answer to the correct number of significant figures.
3. Energetics and Heat Calculations
Formula: Q = mcΔT and ΔH = –Q ÷ n
Used in calorimetry and thermochemistry. You'll need to:
Use mass in grams (usually water or solution)
Use c = 4.18 J/g°C unless stated otherwise
Convert J to kJ where required
Divide energy change by number of moles to calculate molar enthalpy
4. Ideal Gas Law (HL and SL)
Formula: PV = nRT
P = pressure (Pa)
V = volume (m³)
n = moles
R = 8.31 J/mol·K
T = temperature (K)
Common errors occur in unit conversions: cm³ → m³ (÷ 1,000,000), and °C → K (+273).
5. Graphs and Gradients
Required in both the Data Booklet and Internal Assessment. You’ll need to:
Plot experimental data accurately
Interpret linear and non-linear graphs
Calculate gradients and intercepts
Understand what the gradient represents (e.g. rate, k, Ea)
6. Significant Figures and Decimal Places
You’re expected to:
Match the number of sig figs to the least precise value in the calculation
Use scientific notation for very large or small numbers
Round consistently and explain your reasoning in IA or Paper 2
7. Acids and Bases (SL & HL)
Formula: pH = –log[H⁺]
Also: [H⁺] = 10^–pH
This is calculator-based, but you must:
Understand the logic behind logarithms
Use inverse log functions
Manipulate pH and [H⁺] to solve for concentration
HL students will also work with:
pKa and Ka: pKa = –log Ka
Buffers
Strong vs weak acid/base behaviour
8. Equilibrium Constants
Formula: Kc = [products]^coeff / [reactants]^coeff
HL students must also work with:
Kp for gas-phase equilibria
Calculations using ICE tables (Initial, Change, Equilibrium)
Units for Kc and Kp based on the balanced equation
9. Electrochemistry (HL)
Key skills include:
Calculating standard cell potentials: E°cell = E°red – E°ox
Using half-equation values from the data booklet
Interpreting feasibility based on sign of E°cell
10. Reaction Kinetics and Arrhenius Equation (HL)
Formula: ln k = –Ea/R × 1/T + ln A
You’ll need to:
Plot ln k vs 1/T and find the gradient
Calculate activation energy using R = 8.31
Use calculator functions for ln and exponential values
Bonus: Uncertainty and Error Analysis (IA Focus)
You must be able to:
Identify sources of random and systematic error
Calculate percentage uncertainty
Combine uncertainties when multiplying or adding values
Use error bars and explain limitations
This is critical for scoring well in the Internal Assessment (IA), especially Criterion C and D.
Study Tips to Master IB Chemistry Maths
Use Your Calculator Fluently
Practise using:
Exponent and log keys
Brackets to prevent order of operations errors
Memory functions for multi-step problems
Practise Past Paper Questions
Use IB past paper questions and the IB Questionbank. Work through:
Short calculation questions
Multi-step stoichiometry or titration problems
Questions that combine calculation + explanation
Keep a Personal Formula Sheet
Even though many formulas are in the data booklet, you’ll remember them better if you:
Write them out yourself
Group them by topic
Add notes on units and when to use them
Learn Through Mistakes
Create a “maths mistakes” logbook. When you get something wrong, write:
What the mistake was
Why it happened
How to avoid it next time
Final Thoughts: Why Mastering Maths Helps You Master Chemistry
You don’t need to love maths to succeed in IB Chemistry—but you do need to respect it.
From titrations to thermodynamics, good calculation skills build:
Exam confidence
Clarity under pressure
The ability to justify and explain your answers
Since so many marks depend on both doing the maths and explaining it clearly, mastering these skills gives you a major advantage—whether you’re aiming for a level 5 or a level 7.
Need Help With IB Chemistry Maths Calculations?
Dr. Marguerite Quinn is a PhD-qualified Chemistry tutor who specialises in helping SL and HL students improve their maths confidence and achieve top grades.
👉 Book a 15 mins consultation to explore how online lessons can help you strengthen your calculation skills and prepare for your final exams.


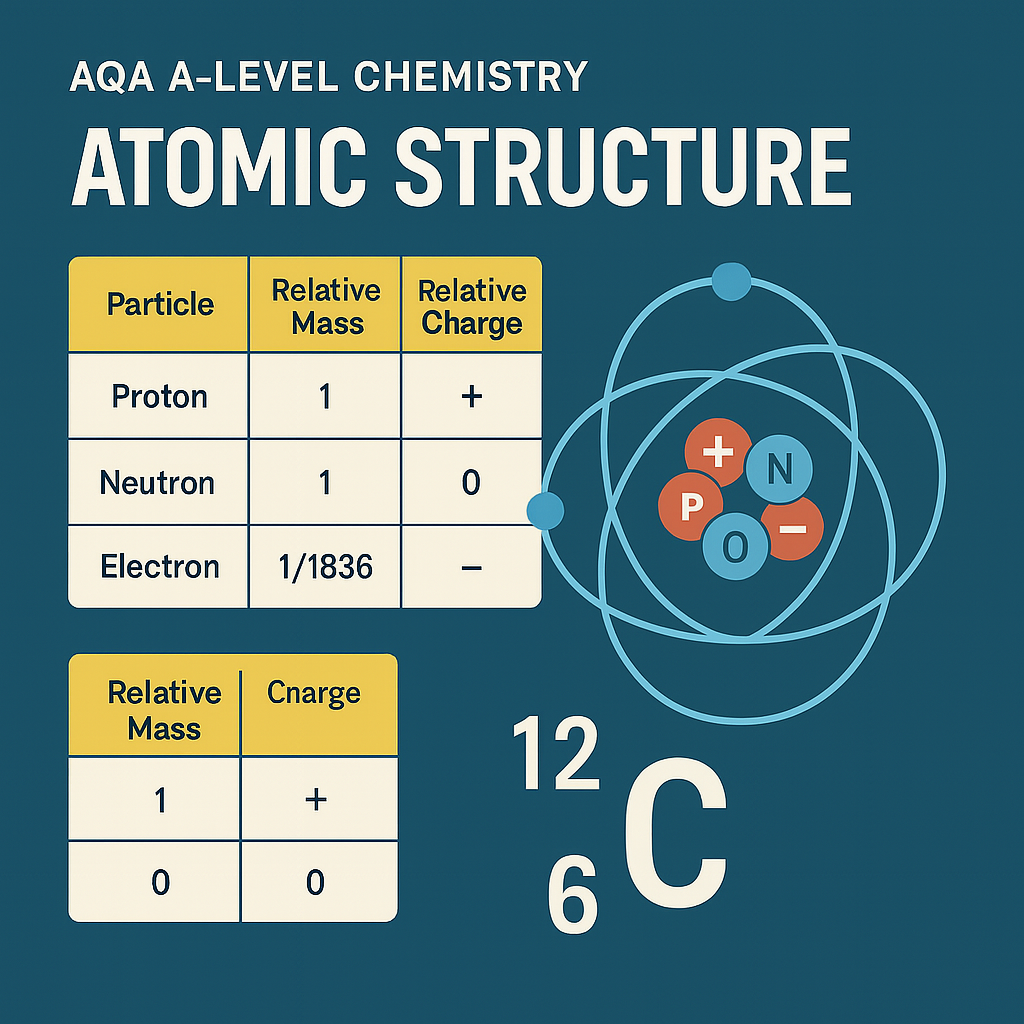


Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.