Understanding the Periodic Table at A-Level: What’s New Beyond GCSE
At A-Level the periodic table becomes a framework for theory, trends, and deep understanding of atomic structure and bonding.
For many students, the periodic table is familiar territory. You first encounter it in Key Stage 3, explore it more deeply at GCSE, and by the time you reach A-Level, you might assume you already understand it.
But here’s the truth: the periodic table at A-Level is a completely different beast.
While GCSE lays the foundation, A-Level Chemistry builds a far more detailed and theoretical framework. It doesn’t just ask what happens—it asks why, how, and under what conditions.
In this guide, we’ll walk through how the periodic table is used at A-Level, what new concepts you need to master, and how to connect your GCSE knowledge with A-Level expectations.
What You Already Know from GCSE
Let’s start by reviewing the key ideas students are expected to know by the end of GCSE:
The periodic table is arranged in order of increasing atomic number
Elements in the same group have the same number of outer electrons
There are repeating patterns (periodicity) across periods
You can predict reactivity and ion formation based on group number
The table is divided into metals (left) and non-metals (right)
Group 1 = alkali metals (more reactive as you go down)
Group 7 = halogens (less reactive as you go down)
Group 0 = noble gases (inert due to full outer shells)
Transition metals = coloured ions, variable oxidation states, catalysts
This level of understanding is focused on observation and prediction. It helps you interpret experimental results and basic reactions.
What Changes at A-Level
At A-Level, the periodic table becomes far more than a reference chart. It becomes a framework for theory, trends, and deep understanding of atomic structure and bonding.
Here’s what you now need to grapple with:
The periodic table as a map of electron configuration patterns
Periodicity explained in terms of effective nuclear charge and shielding
More complex explanations for trends in atomic radius, ionisation energy, electronegativity
d-block chemistry and coordination compounds
Transition metal chemistry and ligand substitution
Group chemistry (particularly groups 2 and 7) with detailed reaction knowledge
Advanced ion formation and oxidation state logic
Understanding how periodic trends affect bonding, acidity, reactivity, and solubility
Let’s break it down section by section.
Electron Configuration and Shells
At GCSE, you may have learned shorthand like:
Sodium = 2.8.1
Chlorine = 2.8.7
At A-Level, you must write full electron configurations using subshells:
Sodium = 1s² 2s² 2p⁶ 3s¹
Chlorine = 1s² 2s² 2p⁶ 3s² 3p⁵
You also learn:
The order of filling (1s, 2s, 2p, 3s, 3p, 4s, 3d, etc.)
How to apply the Aufbau principle, Pauli exclusion principle, and Hund’s rule
Why 4s fills before 3d—and empties first in transition metals
This sets the stage for understanding periodic trends, bonding, and transition metal behaviour.
Ionisation Energy
At GCSE, students learn that:
Ionisation is the removal of an electron
Group 1 elements lose electrons easily
Group 7 elements gain electrons to form negative ions
At A-Level, you must:
Understand first, second, and successive ionisation energies
Know the factors affecting ionisation energy:
Nuclear charge
Distance from nucleus
Electron shielding
Use data to identify element positions in a period or group
Explain anomalies in trends (e.g. the dip between Mg and Al due to orbital differences)
You may even be asked to sketch or interpret a graph of successive ionisation energies—something never covered at GCSE.
Periodicity at a Deeper Level
You already know that properties repeat across periods.
Now you must explain:
The trend in atomic radius across a period: decreases due to increasing nuclear charge
Ionisation energy across a period: generally increases, with blips explained by subshell structures
Melting point trends: e.g., increase in melting point from sodium to silicon (metallic bonding → giant covalent), then sharp drop to simple molecular structures like phosphorus and sulfur
Electrical conductivity trends
Group comparisons: e.g., comparing bonding and boiling points of P₄, S₈, and Cl₂
At A-Level, you're expected to explain these using underlying atomic theory, not just memorise facts.
Group Chemistry: Deeper Reactions and Trends
At GCSE, you memorised trends:
Group 1 gets more reactive down the group
Group 7 gets less reactive
Group 0 is unreactive
At A-Level, you must:
Write balanced ionic and half equations
Explain group 2 reactions with water, oxygen, acid, and thermal decomposition
Describe how solubility changes for sulfates and hydroxides down group 2
Interpret precipitation reactions
Understand redox changes and oxidation states
Recall detailed reactions of halogens with cold and hot alkali
Study disproportionation (e.g., chlorine + water)
Use oxidation states to identify redox reactions across the table
In short, A-Level Chemistry expects you to understand reactions in terms of electrons, bonding, and thermodynamics.
Transition Metals and the d-Block
At GCSE, this is simplified:
Transition metals are strong, conductive, and colourful
They form variable ions and act as catalysts
At A-Level, transition metals become one of the most complex and exciting areas:
You learn about d-orbitals, splitting of energy levels, and coloured complexes
Ligands, coordination numbers, and naming complexes
Ligand substitution, precipitation, and redox reactions
Stability constants (Kstab) and entropy factors
Redox potentials and electrode potential trends
You’re also expected to draw or interpret 3D diagrams of complex ions, and use them to explain colour, shape, and reactivity.
Oxidation States Across the Periodic Table
While GCSE students are expected to identify simple ion charges (e.g. Na⁺, Cl⁻, Mg²⁺), A-Level students must:
Assign oxidation states in complex compounds
Recognise disproportionation
Balance full and half redox equations in acidic and alkaline conditions
Link oxidation state changes to colour changes in redox titrations (e.g. KMnO₄, Cr₂O₇²⁻)
This demands a far greater confidence with electrons, ionic charges, and redox logic.
The Periodic Table as a Predictive Tool
At GCSE, the periodic table helps you predict:
Basic reactions
States of elements
Likely ion charges
At A-Level, it becomes a tool for:
Predicting the outcome of multi-step reactions
Estimating bond types and electronegativity differences
Predicting acid-base behaviour of oxides across a period
Understanding patterns in acidity, solubility, and bonding types
You're expected to use the periodic table in combination with:
Thermodynamic data
Electrode potentials
Solubility rules
Electronic configuration knowledge
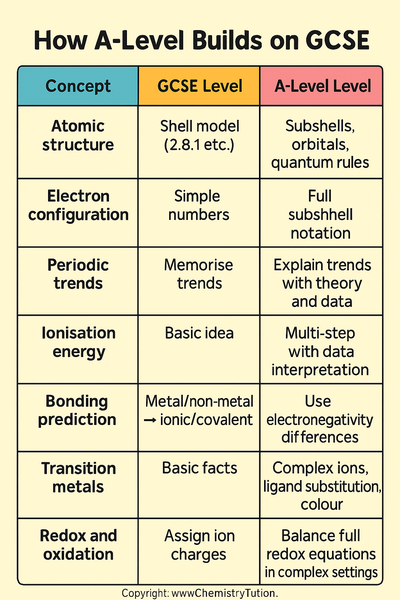
How A-Level Builds on GCSE
How A-Level Builds on GCSE
How to Use the Periodic Table in A-Level Exams
The periodic table is still printed at the back of every exam paper—but you’re expected to use it much more cleverly. Here’s how:
1. Predict Electronic Configurations
You’ll often be asked to write or interpret configurations, especially for ions:
e.g. Cr³⁺ = [Ar] 3d³ (not 4s² 3d¹!)
Recognise exceptions for chromium and copper
2. Interpret Ionisation Energy Graphs
You may get data for successive ionisation energies and need to:
Identify group number
Explain big jumps
Link to electron shells
3. Understand Trends in Bonding and Melting Points
You might be asked:
Why Si has a higher melting point than Cl₂
Why Al is a better conductor than Mg
How structure and bonding change across a period
4. Analyse Complex Equations
You’ll be expected to:
Identify oxidation states
Balance redox reactions
Explain colour changes in transition metal reactions
5. Apply Periodic Trends to Synthesis or Organic Questions
You’ll also use periodic understanding when:
Choosing reagents
Predicting acid-base reactions
Explaining why an intermediate is formed or stable
How to Revise the Periodic Table for A-Level
Make summary sheets for group trends, electronic configurations, and ionisation energy
Use colour to mark transitions, blocks, and patterns
Create flashcards for common ion charges and complex ion names
Practice data questions from past papers
Explain trends aloud—don’t just memorise them
Connect theory to reactions—especially in group and transition metal chemistry
Final Thoughts: The Periodic Table as a Thinking Tool
At GCSE, the periodic table is a guidebook.
At A-Level, it becomes a map of chemical theory—a model for understanding, prediction, and explanation. It links everything: atomic structure, bonding, thermodynamics, redox, acid-base, transition metals, and beyond.
If you engage with it fully, the periodic table doesn’t just help you pass the exam—it makes you think like a chemist.
Need Help Mastering A-Level Chemistry and Periodic Trends?
Dr. Marguerite Quinn is a PhD-qualified Chemistry tutor who helps A-Level students develop deep understanding of periodicity, atomic structure, and exam technique.
👉 Book a 15 mins consultation to explore how personalised online tutoring can help you turn content knowledge into confident application.


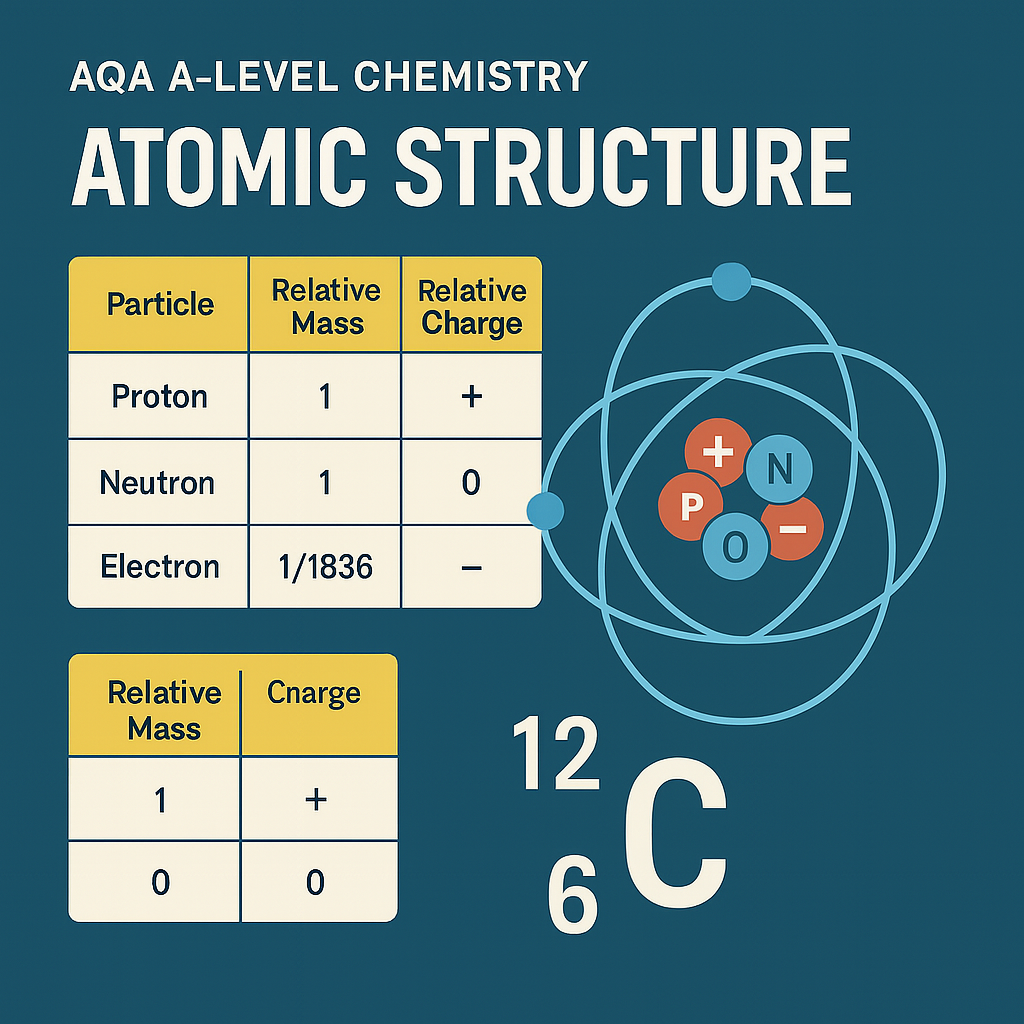


Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.