Understanding the Periodic Table: A GCSE Student’s Guide to Mastering the Elements
The periodic table isn’t just a chart—it’s a tool for thinking.
Whether you're just starting GCSE Chemistry or preparing for your final exams, one tool will come up again and again—the periodic table. It's printed on the inside of your revision guides, it's stuck to classroom walls, and it appears on nearly every Chemistry exam paper.
But despite seeing it constantly, many students don’t fully understand how to use it. The periodic table isn’t just a list of elements—it’s a powerful tool packed with patterns, clues, and essential information.
In this blog, we’ll break down what the periodic table is, how it’s organised, and how you can use it to boost your exam performance and scientific understanding.
What Is the Periodic Table?
The periodic table is a chart that organises all known chemical elements according to their atomic structure and chemical properties. Each element has its own unique place, and this position tells you a lot about how it behaves.
It’s called "periodic" because it shows recurring patterns—certain properties repeat at regular intervals across the table.
Today’s periodic table includes over 100 elements, arranged in a structured way to make Chemistry easier to understand.
How the Periodic Table Is Organised
There are two main ways to understand the structure of the periodic table:
1. Groups (Columns)
The vertical columns are called groups
There are 8 main groups (labelled 1 to 8 or 0, depending on the version)
Elements in the same group have similar chemical properties
They all have the same number of outer shell electrons
Example:
Group 1 (alkali metals): All have 1 electron in their outer shell → highly reactive, especially with water
Group 7 (halogens): All have 7 outer electrons → form -1 ions, very reactive non-metals
Group 0/8 (noble gases): All have full outer shells → very stable and unreactive
2. Periods (Rows)
The horizontal rows are called periods
Each period shows a new energy level (shell) being filled with electrons
As you move across a period, the number of protons and electrons increases
Example:
Period 2 includes lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), and neon (Ne). The atomic number increases by 1 each time, and the properties gradually change.
Key Information in Each Element Box
Each element on the table includes important data:
Element name
Symbol (e.g. H for hydrogen, Na for sodium)
Atomic number (number of protons)
Relative atomic mass (Ar)
Why it matters:
The atomic number defines the element
The Ar helps with mole calculations
The symbol is used in equations and formulas
Tip: The atomic number always increases across the table—but the patterns in reactivity, bonding, and properties are what matter most for GCSE.
Metals vs Non-Metals
There’s a clear divide in the periodic table between metals (on the left and centre) and non-metals (on the right).
Metals are shiny, conductive, malleable, and often reactive
Non-metals are dull, poor conductors, and brittle
The dividing line is usually shown as a diagonal "staircase"
Metalloids (like silicon and boron) lie along the line and show properties of both.
Group 1: The Alkali Metals
Includes lithium (Li), sodium (Na), potassium (K), etc.
Soft metals that can be cut with a knife
Stored in oil to prevent reaction with air or water
React vigorously with water → form hydrogen gas and a metal hydroxide
Key trends as you go down the group:
Increasing reactivity
Lower melting and boiling points
Increasing atomic mass
Why? The outer electron is further from the nucleus → easier to lose → more reactive
Group 7: The Halogens
Includes fluorine (F), chlorine (Cl), bromine (Br), iodine (I), etc.
Non-metals that exist as diatomic molecules (e.g. Cl₂)
React with metals to form salts (e.g. NaCl)
Displace less reactive halogens in displacement reactions
Trends down the group:
Decreasing reactivity
Higher melting and boiling points
Colour becomes darker (chlorine = yellow-green gas, iodine = grey solid)
Why? More electron shells → harder to gain an extra electron
Group 0: The Noble Gases
Includes helium (He), neon (Ne), argon (Ar), etc.
Full outer shells → very stable and unreactive
Used in lights, balloons, and inert environments for welding
Boiling points increase down the group
Key GCSE takeaway: Noble gases do not form compounds easily, and their unreactivity is due to their full outer shell.
Transition Metals
Found in the central block of the periodic table (between groups 2 and 3).
Properties:
Good conductors of heat and electricity
Strong and dense
Can form ions with different charges
Often form coloured compounds
Useful as catalysts (e.g. iron in the Haber process)
Examples of coloured ions:
Copper(II) sulfate = blue
Iron(II) = light green
Iron(III) = yellow/brown
Manganese compounds = purple, pink
GCSE students don’t need to memorise all colours but should recognise the key examples from required practicals and demonstrations.
Periodic Trends to Know for GCSE
Here are some important trends that come up frequently in questions:
1. Reactivity in Group 1 and Group 7
Group 1 becomes more reactive down the group
Group 7 becomes less reactive down the group
This is explained using:
Electron shells
Distance from nucleus
Attraction to outer electrons
Shielding effect
2. Atomic Radius
Increases down a group (more shells)
Decreases across a period (more protons pulling in the same shell of electrons)
3. Melting and Boiling Points
Vary by group
Group 1: Decrease down the group
Group 7: Increase down the group
Transition metals: Usually high
4. Ion Formation and Charge
Group 1: Lose 1 electron → +1 ion
Group 2: Lose 2 electrons → +2 ion
Group 6: Gain 2 electrons → –2 ion
Group 7: Gain 1 electron → –1 ion
Remember: Metals form positive ions, non-metals form negative ions
The History of the Periodic Table
Understanding where the periodic table came from helps you appreciate why it’s so useful.
Dmitri Mendeleev’s Contributions
In 1869, Russian scientist Dmitri Mendeleev created an early version of the periodic table.
What made his table special?
He arranged elements by increasing atomic mass
He grouped elements with similar properties
He left gaps for undiscovered elements
He even predicted the properties of missing elements (like germanium)
Later, when atomic numbers were discovered, the table was adjusted to reflect that order—and all the patterns made even more sense.
How the Modern Periodic Table Was Developed
Today’s periodic table is organised by increasing atomic number (not mass) and reflects the electronic structure of atoms.
It’s divided into:
Metals and non-metals
Groups and periods
Blocks (s, p, d, f) for more advanced Chemistry
But for GCSE, the key idea is: position = prediction. You can predict an element’s:
Reactivity
Ion formation
Type of bonding
Physical and chemical properties
State at room temperature
How to Use the Periodic Table in Exams
Knowing the periodic table isn’t just for show—it can unlock exam marks. Here’s how to apply it:
1. Identifying Unknown Elements
If given a mystery element:
Use its group → number of outer electrons
Use its period → number of shells
Look at position to estimate reactivity, state, and ion charge
2. Writing Electron Configurations
GCSE students often need to write configurations up to calcium (Ca):
Sodium (Na) = 2.8.1
Chlorine (Cl) = 2.8.7
Use the atomic number to divide electrons into shells.
3. Predicting Reactions
If you're given a new Group 1 or Group 7 element, you can still:
Predict its reactivity
Suggest reaction types
Write word or symbol equations
4. Explaining Trends
Questions often ask:
“Explain why potassium is more reactive than lithium.”
“Describe the trend in reactivity of the halogens.”
These are explanation questions. Use:
Electron shells
Distance from nucleus
Electrostatic attraction
Shielding
5. Choosing Methods or Explaining Practical Results
Displacement reactions, colour changes, gas production, pH changes—all these require an understanding of:
Group behaviour
Electron transfer
Reactivity series
Bonding and ion formation
6. Mastering Multiple-Choice Questions
GCSE multiple-choice often includes “Which element has 2 electrons in its outer shell?” or “Which group is chlorine in?”
The periodic table on the exam paper is your friend. Use it.
Tips to Memorise the Periodic Table Basics
You don’t need to memorise every element—but you should know:
Groups 1, 7, and 0 properties
10–15 common element symbols (Na, Cl, O, N, etc.)
How to find atomic number and Ar
Electron configuration basics
Trends and patterns in groups and periods
Examples of common ions and their charges
Use flashcards, diagrams, and interactive games like:
Ptable.com
BBC Bitesize
Kahoot quizzes
Colour-coded periodic tables
Final Thoughts: Master the Periodic Table, Master the Exam
The periodic table isn’t just a chart—it’s a tool for thinking.
When you understand how it works, you gain an advantage in every part of the Chemistry syllabus. You can:
Predict reactions
Explain patterns
Balance equations
Tackle unfamiliar questions
Impress examiners with clear, logical answers
So next time you glance at the periodic table, don’t just skim over it—use it. Learn its logic. And let it guide you.
Need Help with Periodic Table Skills and Exam Confidence?
Dr. Marguerite Quinn is a PhD-qualified Chemistry tutor who helps GCSE and A-Level students build deep understanding of the periodic table, bonding, reactivity, and exam technique.
👉 Book a 15 mins consultation to see how personalised online tutoring can help you achieve the results you need.


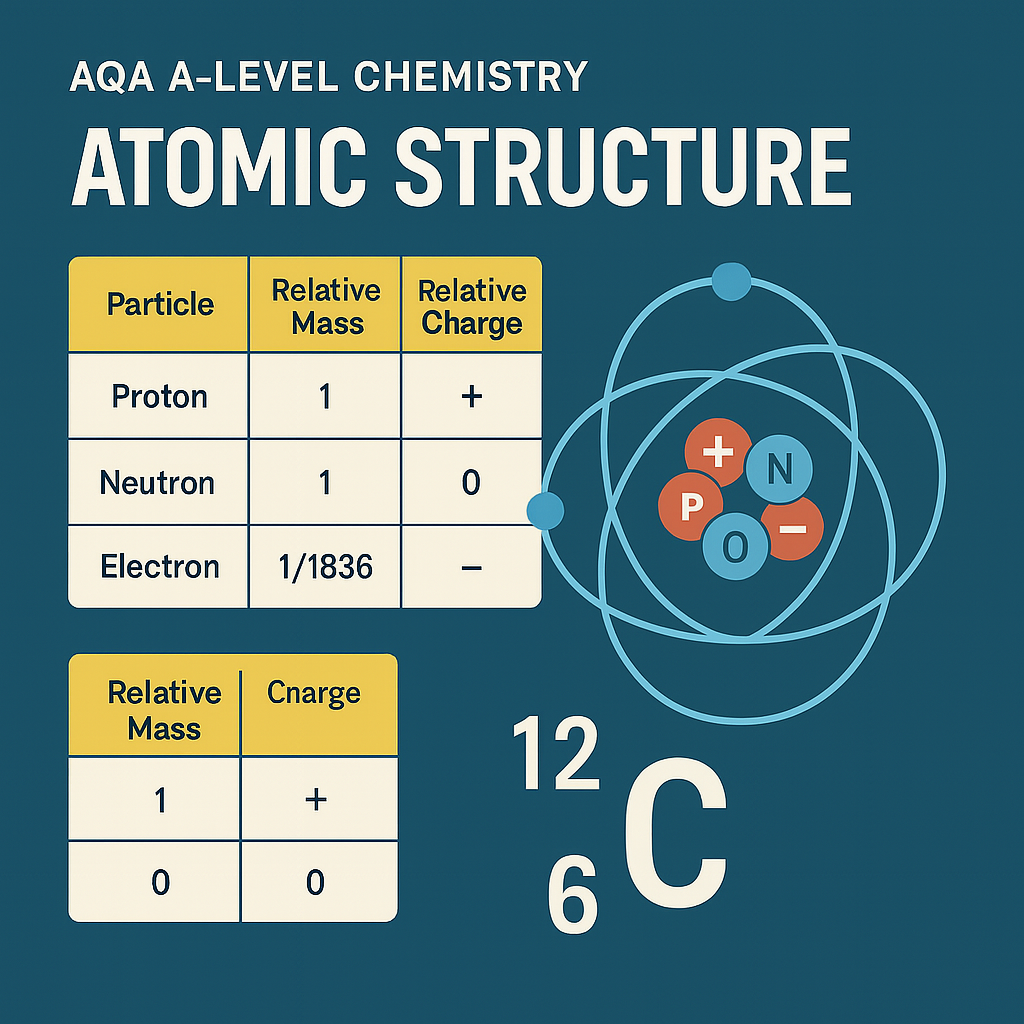


Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.