Understanding Required Practicals: How to Master One of the Most Overlooked Parts of Science Exams
Science is not just about knowing facts—it’s about thinking like a scientist.
When it comes to science exams—especially at GCSE and A-Level—many students focus on theory, calculations, and past paper questions. But there’s one part of the specification that students often overlook until it’s too late: required practicals.
These practicals aren’t just “classroom activities.” They’re a core part of your science course and a major source of exam questions. In fact, exam boards now explicitly assess your understanding of practical methods, data analysis, and evaluation skills.
This blog will explain everything you need to know about required practicals, why they matter, and how to revise them effectively—so you can walk into your exam feeling confident and fully prepared.
What Are Required Practicals?
Required practicals are specific experiments that all students must complete or observe as part of their GCSE or A-Level science course. They are set by the exam board and designed to teach you key practical skills and scientific principles.
Even though you’re no longer assessed on coursework or controlled assessment, you are assessed on these practicals in your written exams.
There are:
10 required practicals in GCSE Chemistry (more if you take Combined Science)
12 required practicals in A-Level Chemistry
Each one is linked to a particular topic and is designed to help you:
Learn core techniques (e.g. titration, chromatography)
Understand variables and measurements
Analyse data and draw conclusions
Evaluate sources of error and improve method design
Why Are Practical Skills Examined?
Science is not just about knowing facts—it’s about thinking like a scientist. That means:
Making observations
Planning investigations
Measuring accurately
Interpreting results
Evaluating reliability
By learning and understanding these practicals, you develop scientific reasoning skills that exam boards want to see in your answers.
Exam boards expect you to recall the method, equipment, and purpose of required practicals—even if you never physically did them.
How Are Required Practicals Assessed in Exams?
You won’t be asked “Describe practical 3 in full.” Instead, you’ll be assessed through application-based questions that test your understanding of:
Methods and procedures
Variables (independent, dependent, control)
Data tables and graphs
Accuracy and precision
Sources of error and uncertainty
Safety precautions
Improvements to experiment design
These questions can be:
Short-answer (e.g. “State one safety precaution”)
Graph-based (e.g. plotting, interpreting gradients)
Six-mark evaluations (e.g. “Suggest how this practical could be improved”)
What Makes Required Practicals Tricky?
Even high-achieving students sometimes struggle with these exam questions. Here’s why:
They don’t revise practicals in the same way as theory
They rely too much on memory, not understanding
They forget key equipment names or procedures
They’re unsure how to describe method improvements
They freeze when faced with an unfamiliar version of a familiar experiment
The good news? Once you understand how to revise practicals properly, you’ll start gaining marks where others lose them.
How to Revise Required Practicals Effectively
Let’s look at a step-by-step approach to mastering practicals.
1. Know the List for Your Exam Board
Start by downloading or printing the list of required practicals for your subject and exam board (e.g. AQA, OCR, Edexcel). For example:
GCSE Chemistry (AQA):
Making salts
Electrolysis
Rates of reaction
Titration (Triple only)
Energy changes
Chromatography
Water purification
Testing gases and ions
A-Level Chemistry (AQA):
Preparation of pure organic solid/liquid
Distillation and reflux
Titration and volumetric analysis
Tests for organic functional groups
Measuring rates of reaction
EMF of electrochemical cells
Buffers and equilibrium investigations
Mark off the ones you’ve done in school—but remember: you’re responsible for knowing all of them, whether or not you did the experiment yourself.
2. Understand the Aim of Each Practical
Each practical is designed to demonstrate a specific concept.
Instead of just memorising the steps, ask:
What’s the scientific principle being investigated?
What would you expect the outcome to be?
How would results support or contradict theory?
Example:
Titration isn’t just about using a burette—it’s about accurately determining the concentration of an acid or alkali.
3. Learn the Method in Clear, Simple Steps
Revise each method in bullet-point format:
Equipment used
How it’s set up
Step-by-step instructions
Any measurements or calculations involved
What to record and why
Practising writing or saying these steps out loud helps commit them to memory. Bonus points if you can also explain why each step is done that way.
4. Focus on Variables and Accuracy
Every practical includes:
Independent variable: what you change
Dependent variable: what you measure
Control variables: what you keep the same
Understanding these helps you:
Plan fair tests
Justify method design
Spot flaws in exam-style questions
You should also revise:
How to make results more accurate
How to reduce uncertainty
How to spot anomalies in results
5. Practise Application and Evaluation Questions
These are often 4- to 6-mark questions that test your understanding, not your memory.
Examples:
“Suggest a control variable and explain why it’s important.”
“Evaluate the method used to measure temperature change.”
“Suggest improvements to increase accuracy.”
“Explain why repeating results is important.”
Use mark schemes to see what examiners are looking for: clear, concise, structured answers using scientific language.
6. Revise Graphs and Data Analysis
You may be asked to:
Plot a graph from data
Interpret a trend
Calculate a gradient (rate of reaction)
Identify anomalies
Comment on precision, reliability, or reproducibility
This is common in:
Rates of reaction
Energy changes
Electrolysis
Titration curves (A-Level)
Practise with real data tables. Use a ruler for graphs. Label axes and units properly. Small errors can cost marks.
7. Use Video Demonstrations to Visualise Setups
If you missed a practical at school, watching a high-quality video is the next best thing.
YouTube channels like:
FreeScienceLessons
FuseSchool
Cognito
AQA / Edexcel official videos
These show the equipment, technique, and expected results. Pause and take notes as if you were in the lab.
8. Create a Required Practicals Revision Booklet
For each practical:
Write the aim
Method steps
Equipment list
Key variables
Example results
How to improve the method
Sample exam questions
You’ll end up with a go-to booklet that’s perfect for last-minute revision.
Bonus: Create flashcards for terminology (e.g. “What is a control variable?” or “Define ‘reproducibility’”).
9. Practise Writing Clear Experimental Plans
At A-Level, you may be asked to design your own experiment to test a hypothesis.
To prepare:
Practise outlining methods from scratch
Include all variables, equipment, and data collection methods
Write in past tense and third person
Be specific (e.g. "Use a 100 cm³ conical flask," not "Use a flask")
This is also excellent preparation for your A-Level Chemistry practical endorsement or NEA (non-exam assessment) if applicable.
10. Don’t Skip Evaluation Skills
Evaluation isn’t just about saying “the experiment could be improved.” It’s about:
Identifying specific errors or uncertainties
Explaining how these affect results
Suggesting realistic, practical improvements
Example:
“Measuring temperature change with a thermometer has a resolution of ±0.5°C. Using a digital thermometer would increase precision.”
These points often come up in higher-mark questions. Practise writing them using real practical examples.
How Are Practicals Marked in Exams?
While you won’t write full lab reports in the exam, your practical understanding is tested in both:
Paper 1 and 2 at GCSE
All A-Level Chemistry papers (often Paper 3 includes practical focus)
Typical marks may come from:
Interpreting data (2–4 marks)
Describing method (2–6 marks)
Evaluating results (4–6 marks)
Designing an experiment (6 marks or more at A-Level)
Marks are awarded for:
Scientific accuracy
Logical structure
Use of technical vocabulary
Clarity and precision
Why Understanding Required Practicals Gives You an Edge
So many students ignore practicals until the last minute. That means:
They miss out on easy marks
They feel less confident
Their answers are vague or unclear
By investing just a little time each week to review required practicals, you can:
Strengthen your understanding of key topics
Score more marks on applied questions
Build confidence and exam technique
Stand out with thoughtful, well-structured answers
Final Tips for Required Practical Mastery
Make a checklist of all practicals and tick them off as you revise
Don’t just read—write, speak, draw, and teach the methods
Learn common errors and how to improve them
Use exam board resources and real questions
Practise, review, repeat—just like theory revision
Need Help with Required Practicals and Exam Skills?
Dr. Marguerite Quinn is a PhD-qualified Chemistry tutor with over 3,470 hours of experience helping students prepare for GCSE and A-Level Chemistry exams—including mastering practicals, applying theory, and writing exam-ready answers.
👉 Book a free consultation to discuss how tailored online lessons can help you feel confident and prepared for any practical-based exam question.


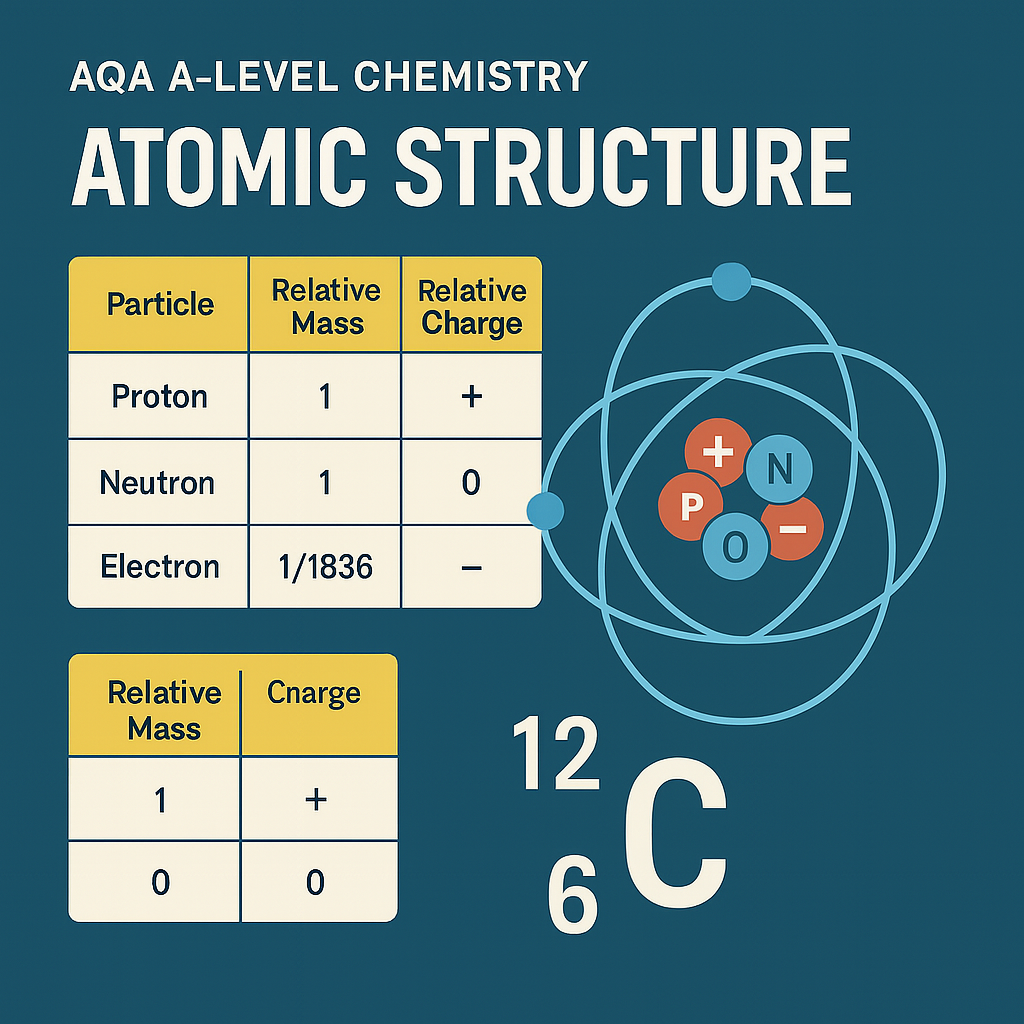


Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.