Understanding the Basics: AQA A-Level Chemistry 3.1.1.1 – Fundamental Particles
Whether you're a student reviewing the topic or a parent supporting your child, make sure this foundation is solid.
The AQA A-Level Chemistry syllabus begins with one of the most essential building blocks of the subject: fundamental particles.
Found in Section 3.1.1.1 of the Physical Chemistry specification, this topic lays the groundwork for many advanced concepts students will encounter later in the course.
In this post, we’ll explore what’s covered in this section, why it matters, and how students can use it to build essential exam and scientific thinking skills.
What Are Fundamental Particles?
The term “fundamental particles” refers to the subatomic particles that make up atoms. In this section, students focus on:
Protons
Neutrons
Electrons
The AQA specification expects students to understand:
The relative mass and charge of each particle
How these particles are arranged in atoms
The meaning of atomic number, mass number, and isotopes
This section is often familiar to students from GCSE Chemistry or Combined Science, but A-Level dives into greater depth and requires precise, confident application.
Key Content Covered in 3.1.1.1
Here’s a breakdown of what students need to know:
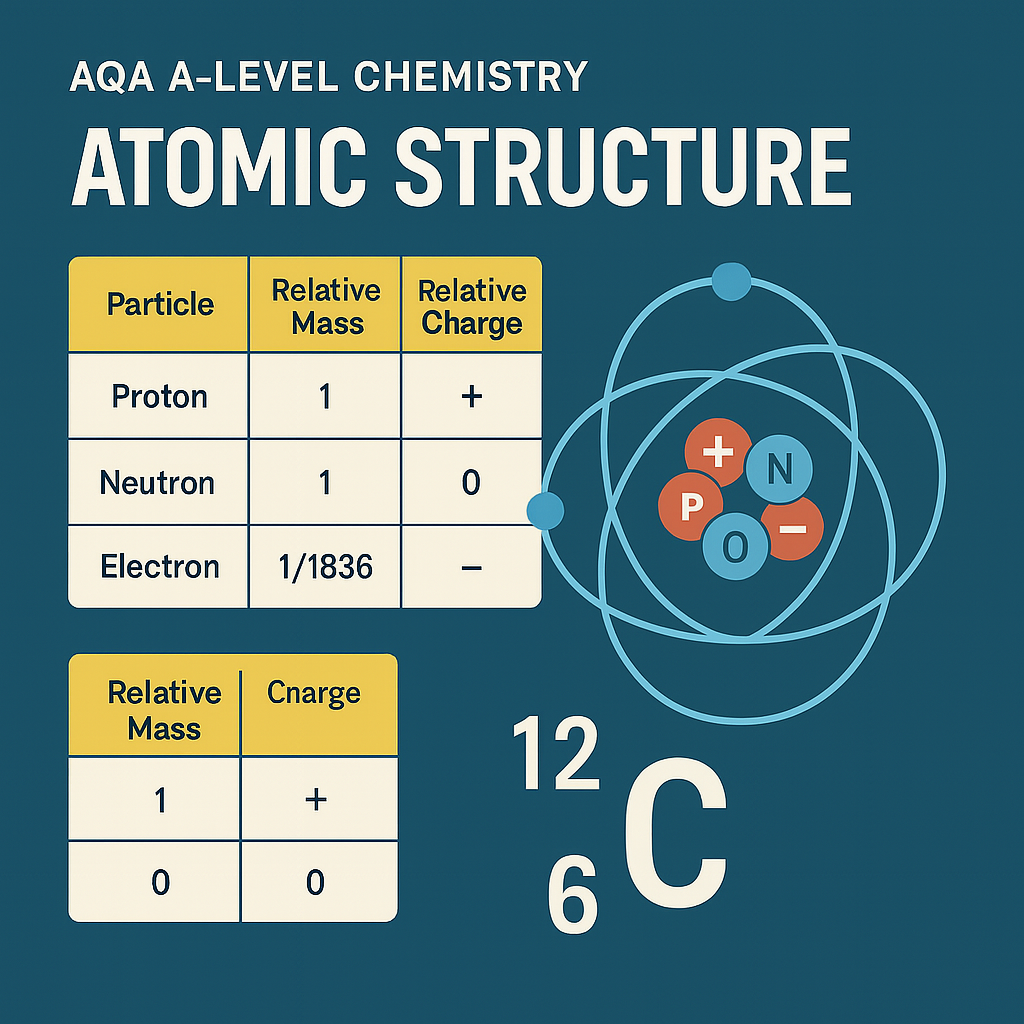
1. The Three Fundamental Particles
| Particle | Relative Mass | Relative Charge |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | 1/1836 | -1 |
Students must be able to recall and apply these values to real atomic scenarios, such as calculating the number of subatomic particles in atoms and ions.
2. Atomic and Mass Numbers
Atomic number (Z) = number of protons
Mass number (A) = protons + neutrons
Students will need to deduce or work out these numbers from isotope notation and apply them to construct atomic and ionic structures.
3. Isotopes
Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. Students should understand:
How to calculate the number of neutrons
That isotopes behave the same chemically but may differ physically (e.g. in mass or radioactivity)
Why isotopic mass is used to calculate relative atomic mass (Ar)
This leads naturally into mass spectrometry in Section 3.1.1.2.
Why This Topic Is So Important
Although this content appears simple, it underpins a huge amount of the A-Level Chemistry course. Without a clear understanding of fundamental particles, students will struggle to:
Balance nuclear equations
Understand ionisation energies and electron configurations
Make sense of periodic trends
Grasp bonding and molecular structure
Interpret mass spectra
It’s a classic example of a foundation topic: easy to overlook, but essential for long-term success.
It’s also an opportunity for students to consolidate numeracy and symbol interpretation — both of which are vital in the AQA exams.
Opportunities for Skills Development
Beyond content, this section helps students develop key chemistry and exam skills.
1. Scientific Literacy and Language
Students must:
Use standard symbols (e.g. ¹²C, ³⁵Cl)
Understand notation used in atomic structures and isotopes
Communicate clearly about mass, charge, and particle relationships
2. Mathematical Skills
Although this topic is qualitative at first glance, it helps students:
Count and calculate subatomic particles
Use simple ratios and subtraction (mass number – atomic number = neutrons)
Apply these calculations to real chemical questions later on
3. Exam Technique
This is a high-yield area in Paper 1 and even in multiple-choice questions. A typical exam task may include:
Describing the subatomic structure of an atom or ion
Explaining why isotopes of an element have the same chemical properties
Determining the number of protons, neutrons, and electrons in an isotope or ion
These questions are often low on content but high on marks — a great opportunity to build early confidence.
4. Conceptual Clarity
Because students often come in with vague or incomplete ideas from GCSE, this is a key moment to correct misconceptions, such as:
Confusing atomic number and mass number
Believing electrons orbit randomly or "outside the atom"
Not recognising how charge affects electron count in ions
A tutor or teacher can help students see these ideas more clearly through visualisation and repetition.
Top Tips for Students Studying This Section
Memorise the table of relative mass and charge. This often appears in quick-mark exam questions.
Practise isotope questions. Especially calculating neutrons and understanding why isotopes are chemically identical.
Don’t skip the basics. These concepts will keep reappearing throughout Year 12 and Year 13 — get them clear early.
Make use of diagrams. Atom diagrams and particle models help make abstract ideas concrete.
Test with mini quizzes or flashcards. These help retain the definitions and data for protons, neutrons, and electrons.
Summary: Building a Strong Foundation
Section 3.1.1.1 of the AQA A-Level Chemistry course might seem simple, but it is one of the most crucial stepping stones for success in the subject. It introduces atomic structure, particle relationships, isotopes, and key numerical skills — all of which form the basis of physical chemistry.
Whether you're a student reviewing the topic or a parent supporting your child, make sure this foundation is solid. A few hours spent mastering this section now will save frustration later when the chemistry gets more complex.
Need help mastering A-Level Chemistry from the ground up?
Book a free 15-minute consultation with Dr Marguerite Quinn to see how one-to-one or group tuition can build confidence, fill knowledge gaps, and support long-term success.




Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.