AQA A-Level Chemistry 3.1.1.2 – What Are Mass Numbers and Isotopes?
Mastering mass numbers and isotopes gives students an edge in calculations, confident terminology use, and interpretation of real-world data.
Section 3.1.1.2 of the AQA A-Level Chemistry syllabus builds on the concept of fundamental particles by introducing two crucial ideas: mass number and isotopes.
While this topic is often introduced at GCSE, A-Level requires students to apply the knowledge more deeply — including in calculations and interpretation of data like mass spectrometry. In this post, we’ll explain what’s covered, why it matters, and how students can use it to boost their exam technique and scientific understanding.
What Is Mass Number?
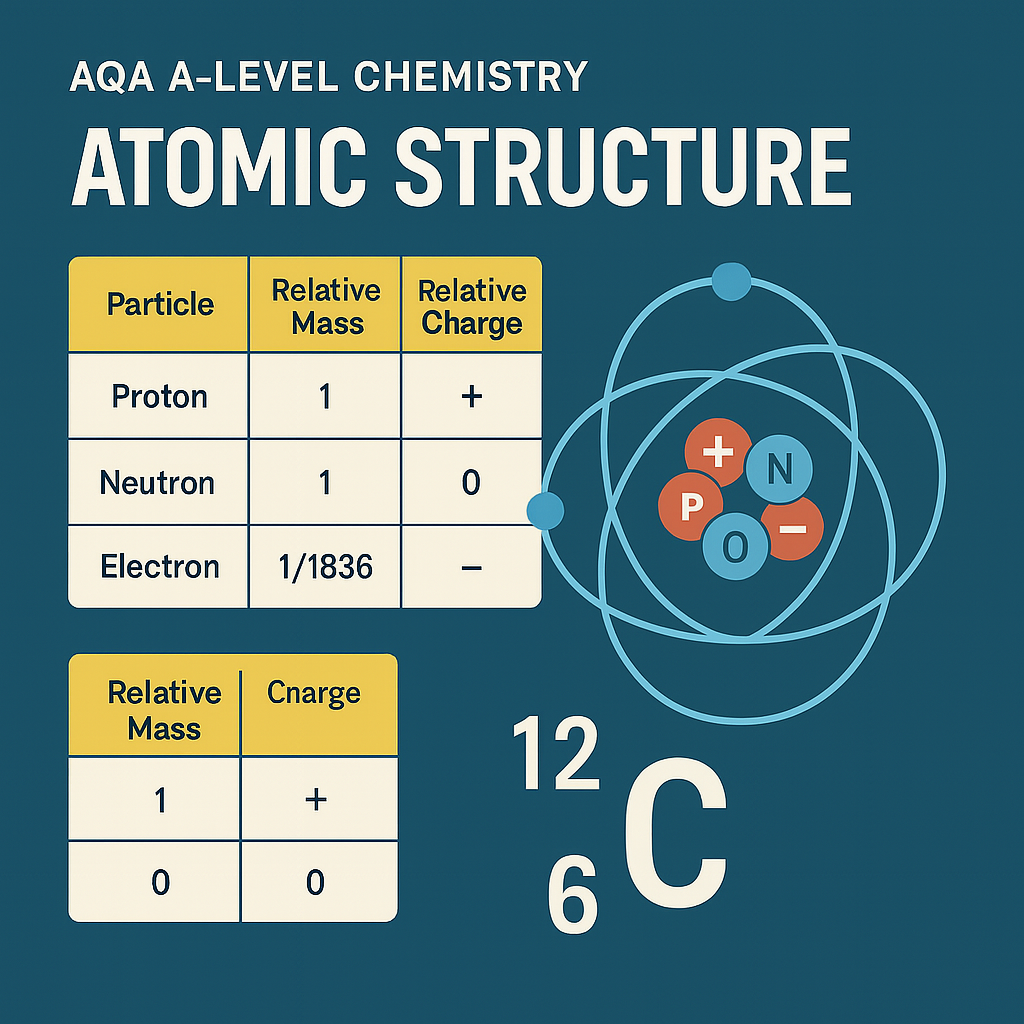
The mass number of an atom is the total number of protons and neutrons in its nucleus.
It is represented by the symbol A, and sits at the top in nuclear notation:
E.g.
¹⁴₆C
Mass number (A) = 14
Atomic number (Z) = 6
Students should be confident in:
Recognising mass number in isotope notation
Using it to calculate the number of neutrons:
Neutrons = Mass number − Atomic number
What Are Isotopes?
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons.
For example, carbon has two common isotopes:
¹²C → 6 protons, 6 neutrons
¹⁴C → 6 protons, 8 neutrons
Both are carbon, because they have 6 protons (atomic number = 6), but they differ in mass number due to extra neutrons.
Key Points Students Need to Know
The atomic number (Z) identifies the element (number of protons).
The mass number (A) tells us the total of protons and neutrons.
Electrons are not included in the mass number.
Isotopes have the same chemical properties (due to same electron configuration) but different physical properties (due to different mass).
Why Is This Topic Important?
Understanding mass number and isotopes is foundational for many areas of chemistry. It directly links to:
Relative atomic mass (Ar)
Mass spectrometry (next topic in the syllabus)
Nuclear equations and reactions
The periodic table – which uses weighted average of isotopes
Radioactive decay (in nuclear chemistry and environmental chemistry)
Without clarity on these definitions and concepts, students will struggle with both calculations and conceptual reasoning in future topics.
Opportunities for Skills Development
Like other early A-Level topics, 3.1.1.2 helps students sharpen key scientific and exam skills:
1. Mathematical Skills
Subtracting atomic number from mass number to calculate neutrons
Interpreting and calculating weighted averages for relative atomic mass (Ar) from isotopic abundances
Rearranging and applying formulas
Using percentage or decimal abundance data
2. Symbol Interpretation
Reading and writing standard isotope notation
Linking numbers to real particle counts
Recognising ions and charged particles
3. Scientific Reasoning
Explaining why isotopes of the same element behave identically in reactions
Applying particle numbers to make predictions about behaviour and trends
4. Exam Technique
Common exam questions include:
“State the number of protons, neutrons, and electrons in…”
“Explain why two isotopes have the same chemical properties.”
“Calculate the relative atomic mass from given data.”
These are often short but high-reward questions that test precision and understanding, rather than extended writing.
How Relative Atomic Mass Links In
Once students understand isotopes, they can appreciate why the Ar on the periodic table is rarely a whole number.
For example:
Chlorine has two common isotopes:
³⁵Cl and ³⁷Cl
If 75% is ³⁵Cl and 25% is ³⁷Cl:Ar = (75 × 35 + 25 × 37) ÷ 100 = 35.5
This kind of calculation often appears in Paper 1 and reinforces concepts introduced here.
Tips for Students Studying This Section
Always subtract to calculate neutrons: mass number − atomic number
Practise Ar calculations with sample data from isotopes
Remember: isotopes = same protons, different neutrons
Use visual models to see how different isotopes of the same element are structured
Know your terminology — don’t confuse atomic number and mass number
Table: Quick Reference for Isotope Notation
SymbolAtomic Number (Z)Mass Number (A)Neutrons¹²C6126¹³C6137¹⁴C6148
Summary: A Key Link in Atomic Understanding
Section 3.1.1.2 may be short, but it unlocks a deeper understanding of the structure of matter. It’s the bridge between simple atomic theory and more advanced applications like relative mass, spectroscopy, and radioactivity.
Mastering mass numbers and isotopes gives students an edge in calculations, confident terminology use, and interpretation of real-world data. It’s an excellent chance to consolidate their early progress at A-Level and build toward success.
Need help tackling tricky chemistry concepts like this?
Book a free 15-minute consultation to see how tailored tutoring — one-to-one or small group — can help you master AQA Chemistry with confidence.




Understand AQA A-Level Chemistry Section 3.1.1.2 on mass number and isotopes. Learn key definitions, isotope notation, calculations, and how this topic builds your scientific and exam skills.